According to the Journal of Physical Chemistry C, American scientists have managed to develop a method of producing graphene out of ordinary acetylene. The new method is not only much simpler existence, but also significantly reduces the production of graphene, allowing to obtain a final product with a minimum amount of impurities.
As you know, graphene is a material with a thickness of one carbon atom. It is highly durable and unique electrochemical properties. The mass use of graphene prevents a huge resource-intensive production of the material. Moreover, attempts to grow graphene from carbon atoms not yet come to fruition. But scientists from the Georgia Institute of Technology, in Atlanta, went a slightly different way. They decided, once the basic structure of graphene is the carbon — so you need to use raw materials with high content of this element. As stated by one of the authors of the project from Landman Uzi,
“Since graphene is composed of carbon, we tried to get it using the simplest carbon molecules. As it turned out, the invisible molecules of ethylene really can turn into a giant graphene sheets. We also managed to solve the problem of the high cost of production, using two innovations in its approach – a catalyst based on noble metal rhodium and the heating of the feedstock to very high temperatures”.
The fact that with the gradual heating up to 700 degrees Celsius in the presence of a catalyst molecule of natural gas ethylene is able to turn into graphene sheets. By the way, fun fact: from ethylene is now doing all the familiar plastic bags, consisting, as you might guess, polyethylene. And the production of polyethylene at the dawn of the chemical industry was also not cheap.
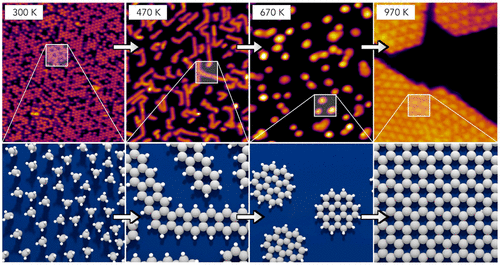
But back to the development of Americans in the course of heating, the scientists, special reaction occurs with catalyst rhodium. First, the gas turns into molecules similar in structure to benzene, and then these molecules are combined with each other to displace hydrogen and form the structure of graphene. The thus obtained graphene is almost 100% pure, as during the heating of the “extra” hydrogen, as it “boils away” from the connection.
At the moment, remains only one problem: after completion of the reaction, the resulting graphene remains connected to the catalyst is rhodium plated, which prevents the use of the substance in the industry. In the plans of the research group of Dr. Landman now just includes the development of the method of separating graphene from rhodium.
Found a way of turning natural gas in graphene
Vladimir Kuznetsov