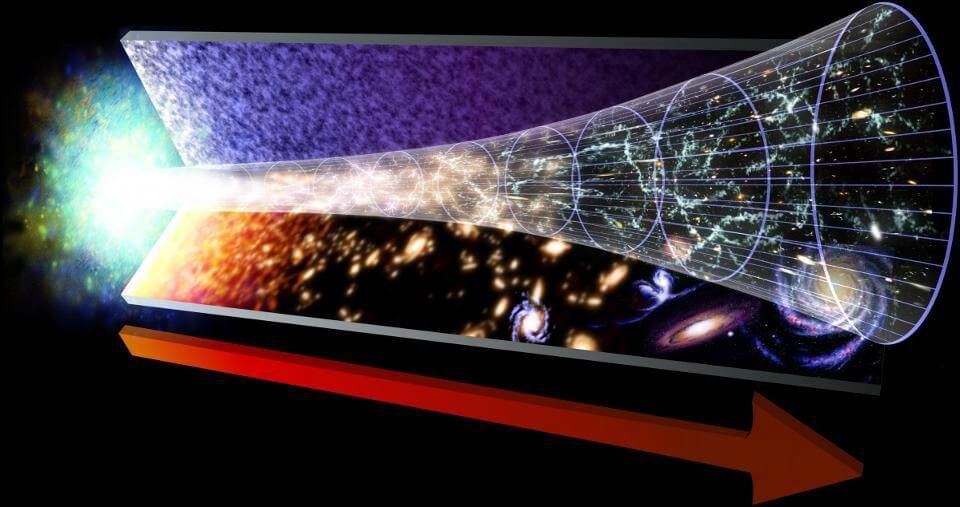
Each passing moment takes us from the past through the present into the future, and there’s no way back: the time always flows in one direction. It is not in place and does not go in reverse; arrow of time always points forward for us. But if we look at the laws of physics — from Newton to Einstein, from Maxwell to Bohr, Dirac to Feynman, they will appear symmetric with respect to time. In other words, the equations that govern reality do not take into consideration which way time goes. Solutions that describe the behavior of any system and obey the laws of physics will be equally good for time backward and time forward. But we, for some reason, know only one direction of time: forward. Where does this arrow of time?
Many people believe that may be a connection between the arrow of time and the amount of entropy. Although most people usually mean by the entropy of “disorder”, this is a very lazy description, and not very accurate. Entropy is a measure of how much thermal (heat) energy can be converted into useful mechanical work. If you have a lot of energy that could potentially work, you have a system with low entropy, and if the energy is small, the entropy of the system is large. The second law of thermodynamics States that the entropy of a closed system can only increase or remain level over time; it will never decrease. In other words, over time the entropy of the Universe must increase. This is the only law of physics which prefers a particular direction of time.
Does this mean that we are experiencing time as it is, only because of the second law of thermodynamics? That there is a fundamental deep connection between the arrow of time and entropy. Some physicists believe that Yes, this could definitely be possible. Perhaps the direction of the arrow of time is directly tied to entropy.
In some cases, the entropy of the perfectly describes the arrow of time, for example, why coffee and milk can be mixed up, but not “blend” back; why ice melting in a warm drink, but does not occur in the cold; why is it broken in the pan the eggs are never going back to presbytie and raw. In all these cases, the initial state of low entropy (with a large number of available, able to work of energy) passed in a state of high entropy (and low available energy) over time. There are many examples of this nature, including a room filled with molecules: on one side a lot of cold and slow molecules on the other many hot and fast. Give yourself time and room will be completely filled with the mixed particles that cannot be divided into two opposing camps. Entropy is increasing. Chaos reigns.
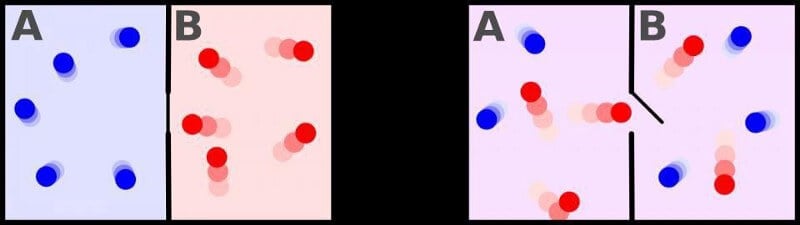
However, perhaps not all that irreversible. Is a pitfall, which you don’t notice many people, learning about the second law of thermodynamics and increasing entropy: it applies only to the entropy of a closed system, or systems, at which nothing acts from without, but the entropy is not decreasing and is not added. This idea was first tried to reveal the physicist James Clerk Maxwell in the 1870-ies: it is enough to have an outside entity that opens the gate between the two sides of the room, allowing the “cold” molecules to flow in one direction, and “hot” in another. This thought has become known as “Maxwell’s demon”, and it actually reduces the entropy of the system.
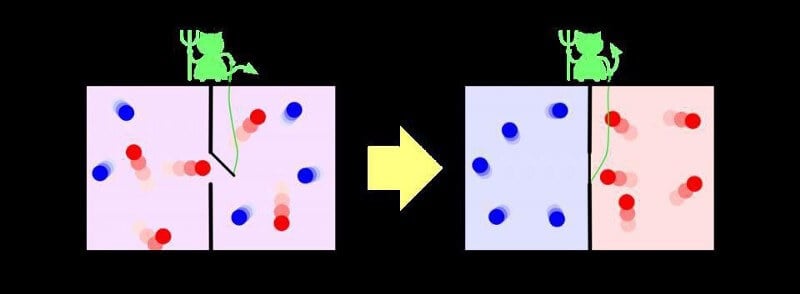
Of course, you don’t violate the second law of thermodynamics in this way. The catch is that the demon will spend an enormous amount of energy on its work to separate the particles. This system, which is in the power of the demon, will be open; if you include the entropy of the demon in the General system of particles, you will find that the total entropy increases. But here’s the trick: even if you lived in a box and did not know about the existence of the demon — in other words, if you lived in this pocket Universe in which entropy decreases — you still would go forward. The thermodynamic arrow of time does not determine the direction in which for us takes time.
So, where does the relationship of the direction of time and our perception? We don’t know. But we know how this thermodynamic arrow of time is not. Our measurements of the entropy of the Universe only know of one huge decrease in the entire history of the cosmos: the end of cosmic inflation and the transition to the Big Bang. (And even this can present a huge increase in entropy, the transition from an inflationary state to the next, filled with matter and radiation). We know that our universe will meet up with cold and empty fate after burn out all the stars will break up all the black holes and dark energy is unrelated’m gonna push them galaxy together. The thermodynamic condition of maximum entropy known as the “heat death” of the Universe. And oddly enough, the state from which our universe emerged — cosmic inflation — has exactly the same properties, only with a much larger rate of expansion than it is now.
Over inflation? As the vacuum energy of the Universe, the energy inherent to empty space, turned into a hot soup of particles, antiparticles and radiation? And if the universe moved from a state of extremely high entropy during cosmic inflation into a state of low entropy during the Big Bang, or entropy during inflation was even lower due to the potential ability of the Universe to perform mechanical work? So far we only have theories; experimental or observational signs that would help us to answer these questions while is not found.
We understand the arrow of time from a thermodynamic point of view, which is incredibly valuable and interesting part of knowledge. But if you want to know why yesterday is unchanged the past, tomorrow is the day, and the present is a moment that you’re living now, thermodynamics will not give you the answer. Nobody will give you.
We still do not understand why time goes only forward
Ilya Hel