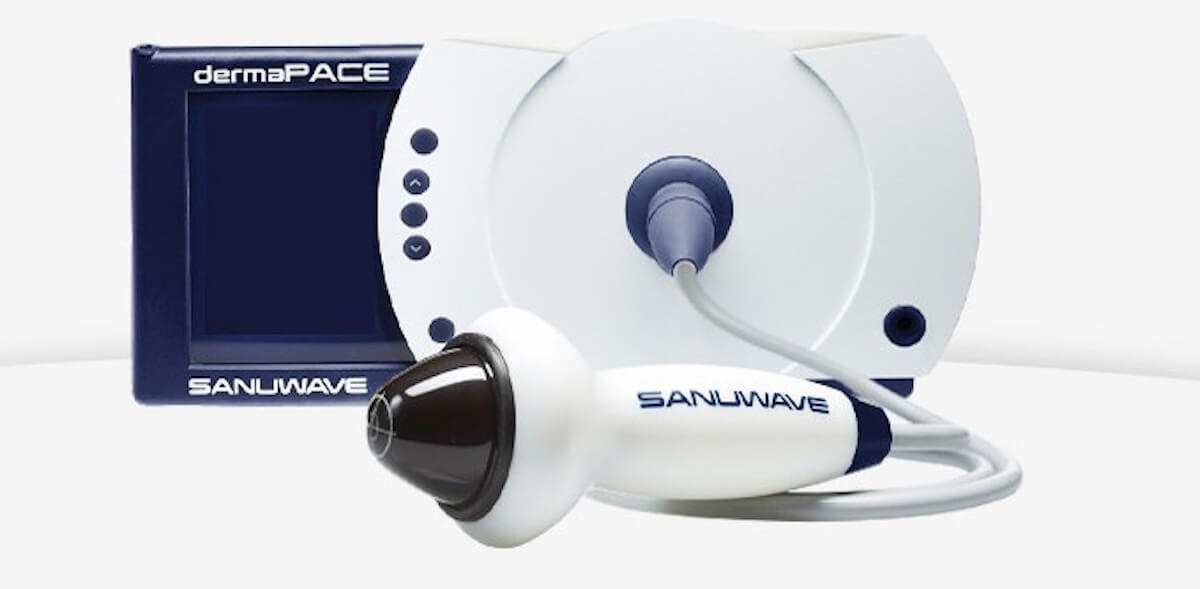
Despite the fact that the method of shock-wave therapy known for a long time, its application was approved is not everywhere. For example, as reported by Engadget, recently the Department of sanitary inspection behind quality of foodstuff and medicines (FDA) approved the first U.S. device for treating wounds using extracorporeal shock wave therapy.
The creation of the device is firm Sanuwave, and developed by medical gagdet Dermapace System is designed to help patients with diabetes. The fact that the majority of diabetics have a complication of long nonhealing trophic ulcers, and multiple clinical trials have shown that acoustic shock waves, 14% improved wound healing. In the recent survey conducted most Sanuwave, participated 336 volunteers. One group of patients received traditional treatment in combination with the device, simulating the treatment of shock waves, but does not have effect. The second group on the background of traditional therapy has at its disposal this unit Dermapace System. After 24 weeks of the experiment found out that in the first group the degree of healing reached 30%, and the second — 44%.
It is worth saying that the use of Dermapace System possible only in combination with conventional drugs for the treatment of ulcers, after the age of 22 years and provided that the wound does not heal self 30 days or more. Not to mention about side effects: participants reported the pain, numbness, headache, fever, nausea, and even infection of wounds. But this outcome is still better than an open wound, which can lead to amputation of the limb.

In USA approved method of treatment by shock waves
Vladimir Kuznetsov