
When you teach the scientific method, you get used to follow a careful procedure to get an idea about a natural phenomenon of our Universe. Start with ideas, experiment, test the idea or disprove it, depending on the result. But in real life everything is much more complicated. Sometimes you spend an experiment and its results differ from what you expected. Sometimes a suitable explanation requires a manifestation of imagination, which goes far beyond the logical judgment of any reasonable person. Today’s physical universe is quite well understood, but the story of how we got to this place, full of surprises. Before you are five great discoveries made in unpredictable ways.
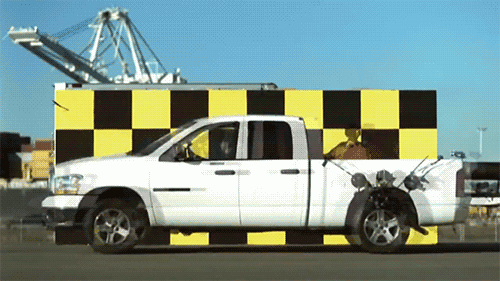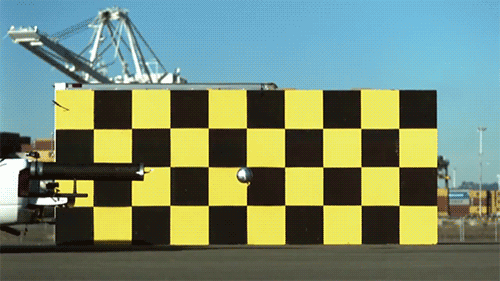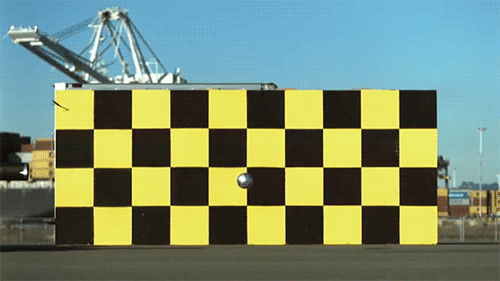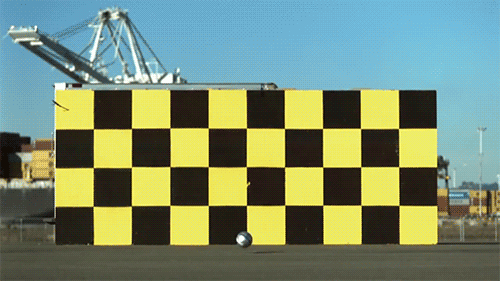
When the kernel crashes from the gun to the back of the truck with exactly the same speed as the moving velocity of the projectile is zero. If it’s light, it always moves at the speed of light.
The speed of light is not changed during acceleration of the light source
Imagine that you throw the ball as far as possible. Depending on what sport you play, the ball can be accelerated to 150 km/h using force of arms. Now imagine that you are on a train that is moving incredibly fast: 450 km/h If you drop the ball from a train moving in the same direction, how fast will the ball move? Just sum velocity: 600 km/h, that’s the answer. Now imagine that instead of having to throw the ball, you emit a beam of light. Add the speed of light to the speed of the train and get the answer which will be… completely wrong.
This was the Central idea of the special theory of relativity, but the actual opening did not Einstein and albert Michelson in the 1880s. And no matter you would produce a beam of light in the direction of the Earth’s movement or perpendicular to this direction. The light always moved with the same speed C, the speed of light in vacuum. Michelson has developed his interferometer to measure the motion of the Earth through the ether, and instead paved the way for relativity. His Nobel prize in 1907 became the most famous in the history of the zero result and the most important in the history of science.
99.9% of the mass of an atom is concentrated in an incredibly dense nucleus
In the early 20th century, scientists believed that atoms are made of the shifts of negatively charged electrons (stuffed cake) enclosed in a positively charged environment (cake), which fills the entire space. Electrons can be torn off or removed, which explains the phenomenon of static electricity. For years, the composite model of the atom in a positively charged substrate, Thompson was accepted. While Ernest Rutherford decided not to test it.
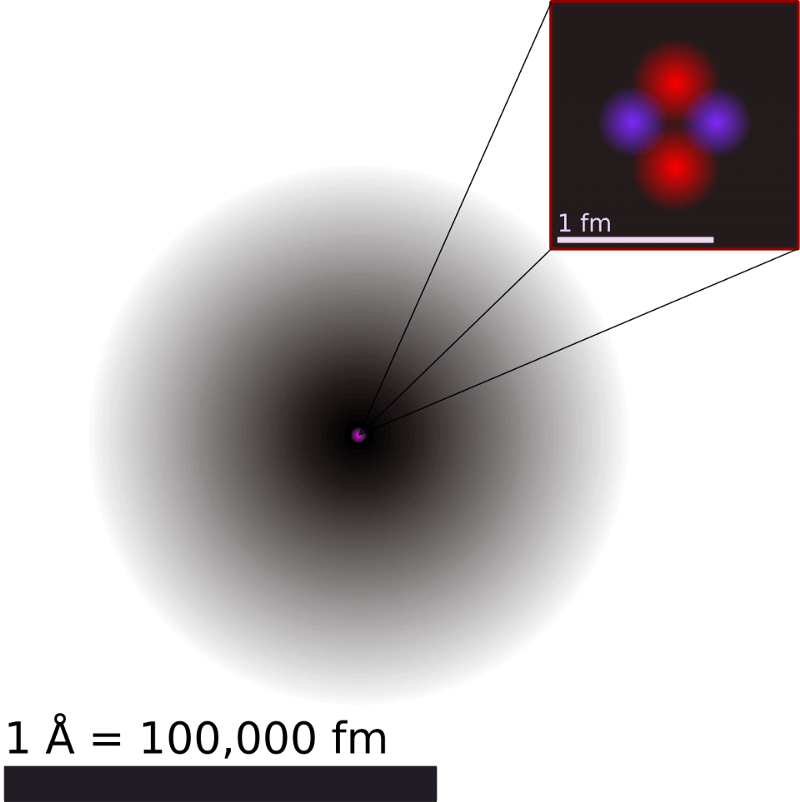
Firing high-energy charged particles (from radioactive decay) a thin plate of gold foil, Rutherford expected all of the particles will pass through. And some of them got through, and some bounced. For Rutherford it was absolutely incredible: if you shot a Cannonball into a tissue, and it bounced.
Rutherford discovered the atomic nucleus, which contained nearly all the mass of the atom is enclosed in a volume that occupies one quadrillion (10-15) the size of the entire atom. This marked the birth of modern physics and paved the way for the quantum revolution of the 20th century.
“The missing energy” has led to the discovery of tiny, virtually invisible particles
In all interactions that we have ever seen between particles, the energy is always preserved. It can be converted from one type to another — potential, kinetic, mass, peace, chemical, nuclear, electrical, etc. — but never destroyed and disappear. About a hundred years ago scientists were puzzled by a single process: in some radioactive decays, the decay products have less total energy than the original reactants. Niels Bohr even postulated that energy is always preserved… except when not. But Bohr was wrong and it took Pauli.
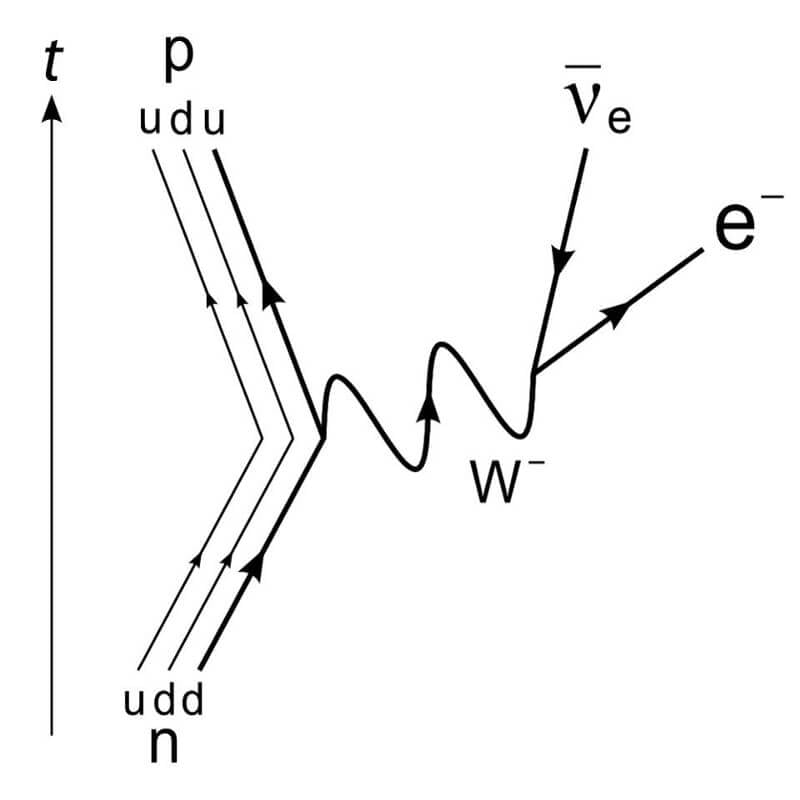
The conversion of a neutron into a proton, electron and neutrino antielectron is a solution to the problem of energy conservation in beta decay
Pauli argued that energy should be saved, and in 1930 he proposed a new particle: the neutrino. This “neutral baby” shouldn’t interact with electromagnetic, and carries a small mass and carries away kinetic energy. Although many were skeptical experiments with the products of nuclear reactions eventually revealed as neutrinos and antineutrinos in the 1950-ies and 1960-ies, which helped to lead physicists as the Standard model and to the model of the weak nuclear interactions. This is a stunning example of how theoretical predictions can sometimes lead to a dramatic breakthrough when suitable experimental methods.
All the particles with which we interact, have high energy, unstable counterparts
It is often said that progress in science does not meet with the phrase “Eureka!” a “very funny”, and this is partly true. If you charge the electroscope, in which two conductive sheet metal connected to another conductor — both sheets will have the same electric charge and the result repel each other. But if you put the electroscope into the vacuum, the leaves should not be discharged, but eventually discharged. How to explain it? The best that came to mind, from space to Earth get high-energy particles, cosmic rays, and the products of their collisions discharged the electroscope.
In 1912, Victor Hess conducted experiments on the search of these high-energy particles in a hot air balloon and found them in great abundance, becoming the father of cosmic rays. Constructing the detection chamber with the magnetic field, you can measure both the speed and the ratio of charge to mass, based on the curves of the movements of the particles. Protons, electrons, and even the first particle of antimatter was discovered with the help of this method, but the biggest surprise came in 1933, when Paul Kunze, working with cosmic rays, found a trace from a particle similar to the electron… only thousands of times heavier.
A muon with a lifetime of only 2.2 microseconds were later confirmed experimentally discovered by Carl Anderson and his student Seth Neddermeyer using cloud camera on the ground. Later it turned out that composite particles (like the proton and neutron) and the fundamental (quarks, electrons and neutrinos) — all have several generations of heavier relatives, the muon is the first part of “generation 2” ever discovered.
The universe began with the explosion, but the discovery was completely random
In the 1940’s George Gamow and his colleagues proposed a radical idea: that the universe, which expands and cools today was hot and dense in the past. And if you go far enough into the past, the universe is hot enough to ionize all the matter in it, and even further — splits an atomic nucleus. This idea became known as the Big Bang, and with her had two serious assumptions:
- Universe, with which we began, was not only a matter of simple protons and electrons, but consisted of a mixture of the light elements that were synthesized in the high-energy young Universe.
- When the universe cooled enough to form neutral atoms, a high-energy radiation was released and began to move in a straight line forever until they hit something, go through the redshift and lose energy with the expansion of the Universe.
It has been suggested that this “cosmic microwave background” will be just a few degrees above absolute zero.
In 1964, Arno Penzias and Bob Wilson accidentally discovered the afterglow of the Big Bang. Working with the radio antenna at bell labs, they found uniform noise everywhere looked to the sky. It was not the sun, the galaxy or the Earth’s atmosphere… they just didn’t know what it is. So they cleaned the antenna, removed the pigeons, but the noise never escaped. And only when the results showed the physics familiar with the detailed predictions of the entire Princeton team, he with the help of the radiometer determined the type of signal and realized the importance of the findings. For the first time, scientists have learned about the origin of the Universe.
Looking back at those scientific knowledge that we have today, with their predictive power, and how centuries of discoveries changed our lives, we are tempted to see in the science of sustainable development ideas. But in fact the history of science is messy, full of surprises and full of spores.
Five unexpected and great discoveries of physics
Ilya Hel