
In the Horse Head nebula discovered something strange. This nebula, named for its silhouette and that is an extended cloud of gas and dust 1500 light years from Earth, where new stars are created. This is one of the most recognizable celestial objects, scientists have studied it well. In 2011, astronomers from the Institute of millimeter radio astronomy (IRAM) and other places again to it.
With 30-meter IRAM telescope in the Spanish Sierra Nevada, they studied two parts of the mane of a horse in the radio spectrum. No, they were not collected image of Horse Head; they were interested in spectrum — they read the light breaking into its component wavelengths, revealing the chemical composition of the nebula. On the screen, these data are similar to the bursts of cardiac monitor; each peak indicates that the molecule in the nebula emit light of a certain wavelength.
Every molecule in the Universe creates its own distinctive signature, based on the position of protons, neutrons and electrons in it. Most of the signatures on the data of Horse Head may be easily explained conventional chemical substances: carbon monoxide, formaldehyde, carbon neutral. But there was also a small unknown on line 89,957 gigahertz. It was a mystery — a molecule, a completely unknown science.
Immediately after receiving these data, Evelyn Ruff from the Paris Observatory and other chemists in its team began to put forward theories on the topic of the molecule, which could create a signal. They concluded that the unknown type must be a linear molecule — the compound in which the atoms are arranged in a straight chain. And only a certain type of linear molecules could produce a spectral fingerprint as seen by chemists. After the list of such molecules, they stumbled on C3H+, propenylidene. This molecular ion had never seen before. In fact, he wasn’t even supposed to exist. And if there were, it would be extremely unstable. On the Ground he almost immediately would react with something else and would have formed a familiar shape. But in space, where pressure is low, and the molecules rarely collide with something with which you can form a relationship, C3H+ may exist.
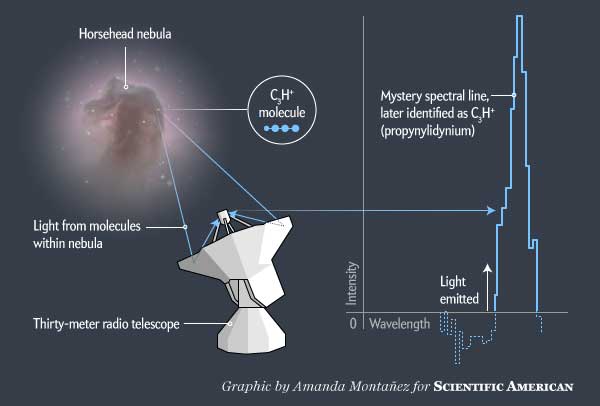
Rueff and her colleagues studied whether the Horse Head nebula to contain the right ingredients and conditions for the formation of this molecule. In 2012 they published a paper in Astronomy & Astrophysics, in which he concluded that the signature found most likely C3H+. “I was relatively confident, says Rueff. — But it took another two or three years to convince everyone that we got it right”.
At first, some skeptics challenged this conclusion — if C3H+ no one has seen before, where they are sure that this is the molecule? The clincher came last year when scientists from the University of Cologne in Germany decided to create for a while C3H+ in the laboratory. They not only proved that the molecule exists, they also allowed the scientists to measure its spectrum and it was the same one that was in the Horse’s Head. “I was pleased to find a molecule, the existence of which we had not thought, says Rueff. — When you can come to that conclusion using logic, you are a real detective”.
One strange molecule was defined, but there are still many such. The Horse Head nebula is no exception. Almost everywhere in the Universe, astronomers where to look — unless, of course, look carefully, they see an uncertain spectral lines. Connection with which we humans are familiar and which create a great variety of materials on this planet, just a part of this created nature. In the end, after decades of development of theoretical models and methods of computer simulation and laboratory experiments on the reproduction of new molecules, astrogemini begin to give names to a number of undefined lines.
Empty space
Most recently, in the 60 years, most scientists doubted that in interstellar space could exist of the molecule — radiation there must be so severe that will not allow to exist something that is more atom pairs or free radicals. In 1968, physicist Charles Townes of the University of California at Berkeley decided anyway to look for molecules in space. “I had a feeling that the majority of Berkeley astronomers thought my idea a little wild,” recalled Townes, Nobel laureate in 2006. But Townes never gave up and built a new amplifier for six-meter antenna radioobservatory Hat Creek in California, which revealed the presence of ammonia in Sagittarius B2 cloud. “How simple and how beautiful. he wrote. Media and academics have started to discuss”.

In subsequent years, astronomers have discovered more than 200 types of molecules floating in space. Many differed greatly from what we have seen on our planet. “Usually we do chemistry on the basis of conditions that are on the Earth, says Ryan Fortenberry, estrogenic Georgia southern University. — When we get away from this paradigm, the chemical can be create without any restrictions. If you imagine a molecule, no matter how strange, there is a certain probability that after the nth time somewhere on the outskirts of the immense space it appears.”
The space is literally a different environment. Temperatures can be much, much higher than on Earth (for example, in the star’s atmosphere), and much, much lower (in a relatively empty interstellar space). Similarly, pressure (high or low) different from the earth. Consequently, molecules that can form in space, the planet may not appear if ever — and if they will, we will have high activity. “The molecule can be years of hanging out in interstellar space, before they encounter another molecule, says Timothy Lee, astrophysics Research center at NASA Ames. — There may be a region without radiation, so even if the molecule is not stable, it will last a long time.”
These space molecules after identification could have much to teach us. Some of them might be useful, if scientists can recreate them in the lab and learn to use their properties. Other molecules can help in the explanation of the origin of organic components that gave rise to life on Earth. They can also expand the boundaries of our knowledge about what is chemically possible in our Universe.
Telescopes that will change everything
In the past decade, when the emergence of powerful new telescopes are able to detect weak spectral lines, the search of foreign molecules accelerated. “Now indeed is the flowering of astronomie, says Suzanne Widicus weaver, the steering group of astrochemical at Emory University. Data that are now available have improved dramatically over ten years. High altitude Observatory NASA SOFIA (stratospheric Observatory of infrared astronomy), mounted onboard a Boeing 747SP, began to observe infrared light and microwave in 2010, and space Observatory the Herschel of the European space Agency went into orbit in 2009 and observes the same wavelengths.

And is really game-changing is Atamanskiy the ALMA telescope, a cluster of 66 radiologic open in 2013. At an altitude of 5,200 meters on the plateau Chajnantor, similar to Mars atkarskoy the desert, the most arid place in the world, the ALMA antennas working in unison, collecting light cosmic objects. Incredibly dark and transparent sky in which practically there are no clouds, the telescope provides unprecedented sensitivity and allow to precisely capture wavelengths from infrared to radio. ALMA creates a visual and spectral picture of each pixel of their images, producing tens of thousands of spectral lines in each area of the observed sky. “It surprises and causes excitement at the same time, says Widicus weaver. — These datasets are so huge that we often have to send their scientists on flash drives so they can download them”. The data stream provides an abundance of new spectral lines, which have to look astronomical. But how uncertain and fingerprints at the crime scene, these lines are useless to scientists, until they realize what kind of molecules they form.
In search of respect
To identify the molecules corresponding to these lines, scientists can go two ways. As in the case of C3H+, astrogemini can start with the theory, using divination on the spectrum to try to guess which molecule may be hiding underneath. Method quantum chemistry ab initio (ab initio Latin for “from the beginning”) allows scientists to start with pure quantum mechanics is the theory describing the behavior of subatomic particles, to calculate properties of molecules based on the movement of protons, neutrons and electrons in atoms and their constituents. On a supercomputer, you can run repeated simulations of molecules, each time slightly adjusting its structure and the arrangement of its particles, and look at the results to determine the optimal geometry of the components. “Quantum chemistry we are not limited that can synthesize, says Fortenberry. — We are limited by the size of the molecules. We need more computational power to make calculations”.
Scientists can also find irrefutable evidence of new molecules, creating them in the laboratory and directly by measuring their spectral characteristics. The General method begins with the gas chamber, through which electricity is passed. The electron current can collide with the gas molecule and break its chemical bonds, creating something new. Scientists support gas at very low pressure, so any new chemical substance has a chance to live a couple of moments before you collide with another molecule and react. The scientists then Shine the camera light of different wavelengths to measure spectrum of what’s inside. “You can be in a position, when produced in the laboratory from the same molecule that exists in space, but I don’t know exactly what, said Michael McCarthy, a physicist from the Harvard-Smithsonian astrophysical center. — So you are left to try to deduce the elemental composition of a combination of different laboratory experiments with different samples”.
In 2006 McCarthy and his colleagues have created a negatively charged molecule, C6H -, and changed its range. Soon after, they found the same spectral imprint in the interstellar molecular cloud of Taurus at 430 light years away. Previous searches of negatively charged particles in space to anything did not lead, therefore, many scientists doubt that they exist in significant volumes. “This has led us to many discoveries, thanks to which we can identify molecules in the laboratory and then in space,” says McCarthy. The team has since found C6H – in many, more than a dozen cosmic sources.
In the 1980s, in an attempt to create new chemicals, scientists have produced a molecule. (36ArH+), weird connection, which is not found on Earth, including hydrogen in the inert gas argon. In 2013 astronomers found Argonia in space, first in the crab nebula, and later in a distant galaxy observations with ALMA. Compounds with noble gases are formed only in very specific circumstances; scientists think that in space high-energy particles — cosmic rays — argon face and knocking him to the electrons, allowing them to connect with hydrogen. For this reason, if scientists see Argonia in some region of space, they believe that this area is awash with cosmic rays. “This is a specific indicator of certain conditions, a very important thing in space,” says Holger müller of the University of Cologne.
New world of molecules
Many of the molecules that lurk in the stars and nebulae, to the extreme strange. Ask how they will look or what will be to touch, is useless, because even if you pick up, they will instantly react. If you still fail to contact them, they will almost certainly prove to be toxic and carcinogenic. Oddly enough, scientists have a rough idea of how it will smell some other people’s molecules: many of them belong to the class of aromatic compounds, benzene derivatives, which were originally shared names with strong odors.
Some of the new compounds demonstrate surprising atomic structure and divide the charge between atoms in a strange way. Sometimes they call into question the modern theory of molecular bonding. A recent example is the molecule SiCSi found in 2015 in a dying star, consisting of two silicon atoms and one carbon atom, which are connected in an unexpected way. The result is such a strange molecule that has a spectral signature different from those that predict a common theoretical model.
Space of a molecule can help us to answer one of the most fundamental questions of the Universe: how life began? Scientists don’t know where it originally emerged amino acids, the building blocks of life on Earth or in space (and after you were brought to our planet by comets and meteorites). The answer to this question can also tell whether many of the amino acids in the Universe and could they theoretically to sow life on myriads of other exoplanets. Astrogemini have noticed signs of the presence of amino acids in space, and the connection of the molecules that underlie them.
Finally, it is possible that some rare species may prove useful if they can be created in large enough quantities and be able to maintain in controlled conditions. “The great hope of astronomie is to find molecules that will possess completely new properties and which we can apply to solve the earth’s problems,” says Fortenberry.
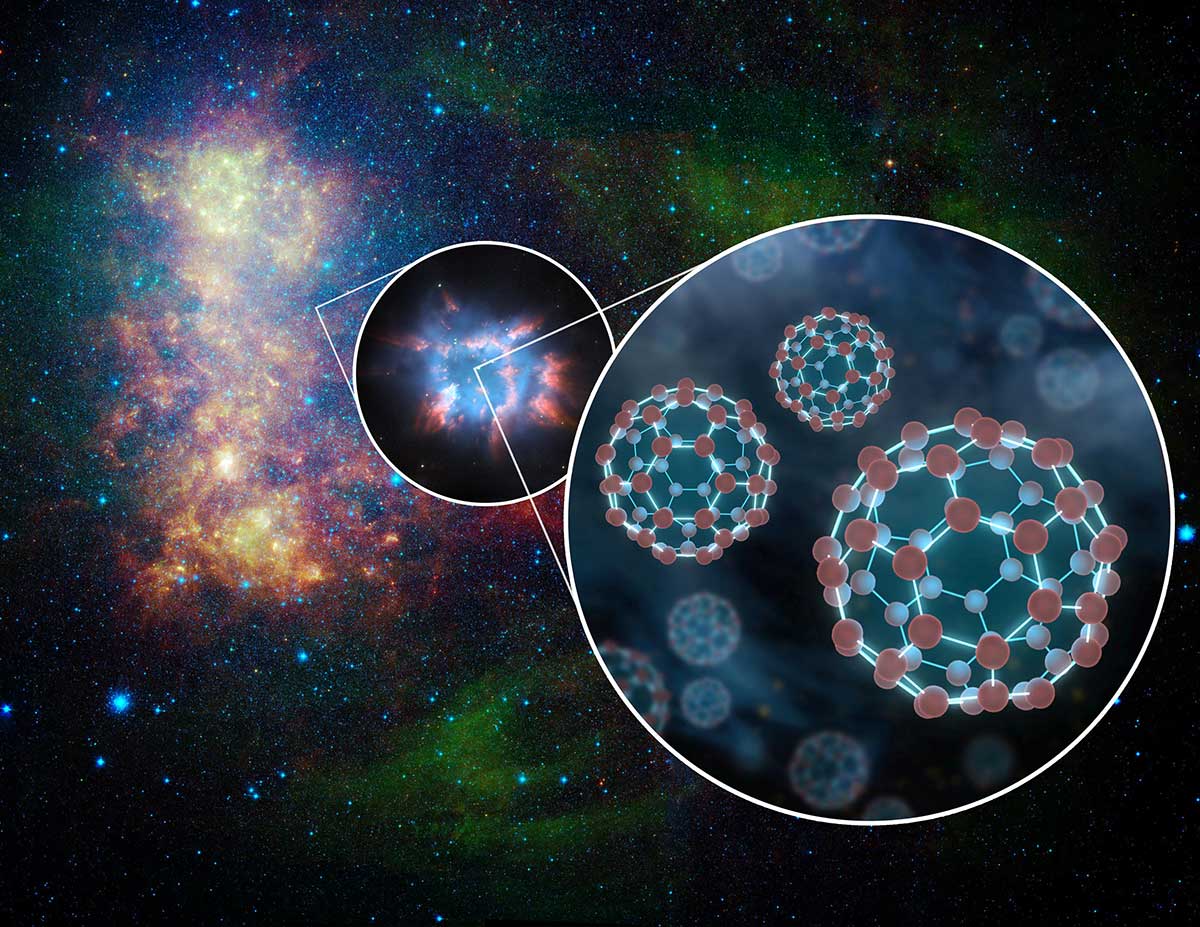
A good example of a molecule “fullerenes”. These large Assembly of 60 carbon atoms were first created in the laboratory in 1985 (and brought a Nobel prize). After almost ten years, astronomers have seen the spectral lines in interstellar gas, which exactly indicated the positively charged version of the fullerenes, and this relationship was confirmed in July, when the researchers compared the signatures with a spectrum of fullerenes that have been created in cosmoshopping conditions in the laboratory. Later fullerenes were not just a strange space discovery, and it is a decent practical tool for nanotechnology, useful for building materials, improve solar panels, and even in pharmaceuticals.
So far, astrogemini still splashing in the shallows of a great sea of molecules somewhere in space. Their findings remind us that our own corner of the space is relatively small — may be small, not significantly, only one example of the possibilities. Perhaps those molecules that we have on Earth, are actually exotic and C3H+, fullerenes and other yet unknown molecules — normal material universe.
