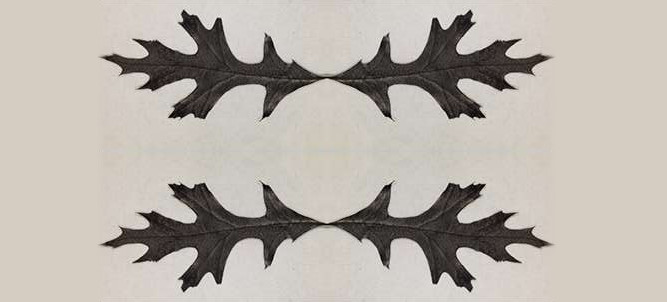
This oak leaf has a secret. Rather than creating energy via photosynthesis, it can pump out electricity—because it’s actually a battery.
Really, it no longer shares that much in common with a leaf you may pluck from a tree: It’s been carbonized, by being held at 1,832°F for an hour. That created a sheet of nanostructured carbon, and the researchers then filled the pores on its lower surface with a sodium electrolyte. Essentially the leaf becomes the anode of the battery, which is capable of storing 360mAh per gram of its weight. The research is published in Applied Materials and Interfaces.
It probably won’t power anything you own, though the researchers are considering whether it could be used as a bulk energy storage technique. Instead, it’s an experiment designed to investigate new ways of using sodium, as opposed to lithium, as an electrolyte. Sodium promises to create batteries that hold more charge, but the resulting cells degrade far faster. It’s thought that new microstructures of carbon—such as those created in the leaf—could help solve that particular problem. Eventually.
[Applied Materials and Interfaces]
